And it works with iPhone!
What you need to know
- Withings has released a new smartwatch.
- The ScanWatch is an FDA-approved smartwatch with lots of medical features.
- It also has 30-days of battery life.
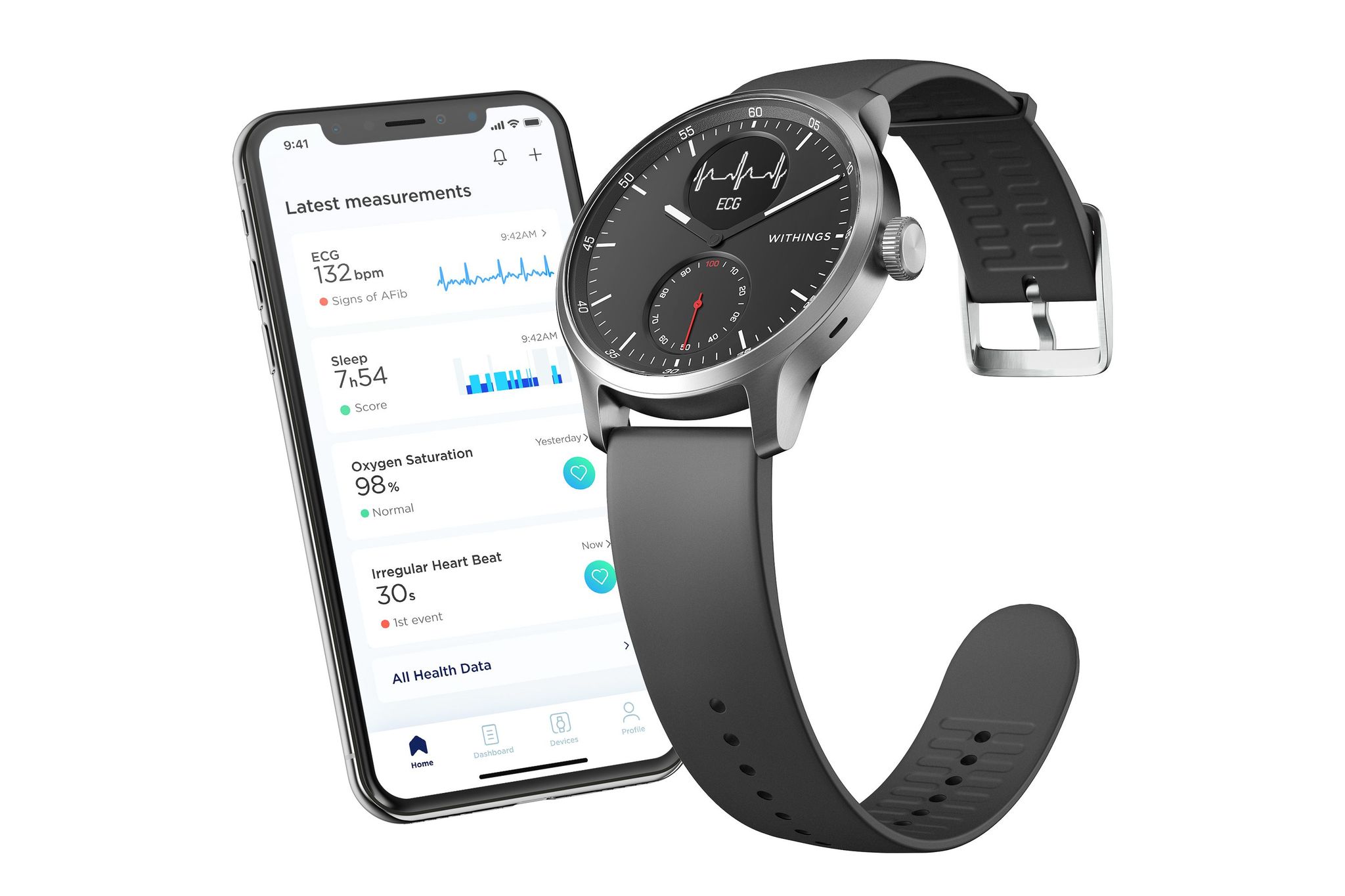
Withings has today released its new ScanWatch, featuring FDA-approved health monitoring and 30-day battery life.
The new watch is the company's "most medical advanced wearable to date" featuring heart-rate monitoring, an ECG, SpO2 measurements for checking blood oxygen, as well as sleep and activity tracking.
In a press release the company stated:
ScanWatch has been FDA cleared to both record ECG and SpO2 measurements. Developed with cardiologists and sleep experts, ScanWatch has been validated in two clinical studies and is designed with a stainless-steel case and durable sapphire glass watch face that combines both analog hands as well as a large digital display. Priced at $279 (38mm) and $299 (42 mm), it comes in a choice of black or white faces and a host of medical monitoring and health tracking capacities.
The new smartwatch is available from Amazon, Withings, and Best Buy from today. It features a heart rate scanner, clinically validated SpO2 monitoring, activity tracking, training mode, fitness level assessment through VO2 Max, sleep monitoring, smartphone notifications, and a 30-day battery life.
Of course, the ScanWatch differs alot from the Apple Watch Series 7 because it doesn't have a full display, but rather a hybrid display with a physical clock face. With that being said a 30-day battery life and clinical health monitoring could be a big draw for some.









0 comments:
Post a Comment